Lithium-ion batteries have long been the dominant power source for electric vehicles and are increasingly considered for renewable energy storage on the electric grid. However, experts project supply shortages of lithium within the next five to ten years due to rapid market expansion.
“Sodium-ion batteries are emerging as a compelling alternative to lithium-ion batteries due to the greater abundance and lower cost of sodium,” stated Gui-Liang Xu, a chemist at the U.S. Department of Energy’s (DOE) Argonne National Laboratory.
Despite the potential of sodium-ion batteries, commercialization has faced significant challenges, particularly the rapid decline in performance of sodium-containing cathodes with repeated charge and discharge cycles.
Researchers at Argonne have made strides in addressing this issue with a new design for a sodium-ion oxide cathode. This design closely follows a previous successful lithium-ion oxide cathode model known for its high energy storage capacity and longevity.
Both designs feature microscopic cathode particles made from a mix of transition metals, which may include nickel, cobalt, iron, or manganese. Importantly, these metals are not uniformly distributed; for instance, nickel forms the core, while cobalt and manganese create a surrounding shell. The manganese-rich exterior enhances structural stability during charge-discharge cycling, while the nickel-rich core provides significant energy storage capacity.
Initial testing of this design revealed a steady decline in the cathode’s energy storage capacity during cycling, which researchers attributed to cracks forming in the particles due to strain at the shell-core interface. To mitigate this issue, the team focused on refining the cathode preparation method to eliminate strain prior to cycling.
The precursor material used in the synthesis is a hydroxide containing oxygen, hydrogen, nickel, cobalt, and manganese. The team produced two hydroxide versions: one with a gradient metal distribution from core to shell and another with an even distribution of metals throughout each particle.
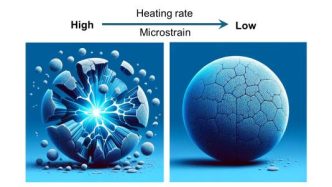
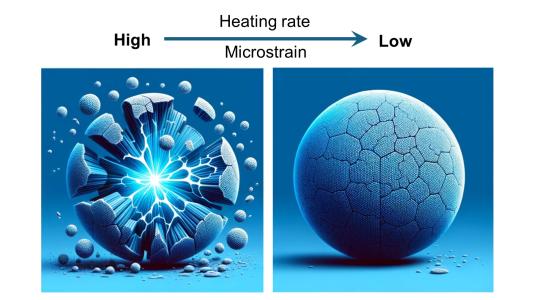
To create the final product, the team heated a mixture of the precursor material and sodium hydroxide to temperatures as high as 600 degrees Celsius, maintaining that temperature for a specified duration before cooling it to room temperature. Different heating rates were also tested.
Throughout this process, the team monitored changes in particle structure using two DOE Office of Science facilities: the Advanced Photon Source at Argonne and the National Synchrotron Light Source II at Brookhaven National Laboratory. Additional analysis was conducted at the Center for Nanoscale Materials at Argonne, and data reconstruction into detailed 3D images was performed using the Polaris supercomputer at the Argonne Leadership Computing Facility.
The initial findings indicated no cracks in uniformly distributed particles; however, cracks emerged in the gradient particles at temperatures as low as 250 degrees Celsius. These cracks originated at the core and core-shell boundary before progressing to the surface, highlighting the significant strain caused by the metal gradient.
“Since we know that gradient particles can produce cathodes with high energy storage capacity, we wanted to find heat treatment conditions that will eliminate the cracks in the gradient particles,” said Wenhua Zuo, an Argonne postdoctoral appointee.
The rate of heating was identified as a critical factor; cracks developed at a heating rate of five degrees per minute but not at a slower rate of one degree per minute. Tests with cathode particles prepared at the slower rate demonstrated sustained high performance for over 400 cycles.
The research team is now focused on eliminating nickel from the cathode to further reduce costs and enhance sustainability.
Funding for this project was provided by the DOE Vehicle Technologies Office, Office of Energy Efficiency and Renewable Energy, and Advanced Scientific Computing Research Program. The research was published in Nature Nanotechnology. Key contributors from Argonne include Wenqian Xu, Gui-Liang Xu, Amine, Jihyeon Gim, Tianyi Li, Dewen Hou, Yibo Gao, Shiyuan Zhou, Chen Zhao, Xin Jia, Zhenzhen Yang, and Yuzi Liu, along with Xianghui Xiao from Brookhaven.