Researchers at Cornell University have engineered a porous crystal that effectively absorbs lithium-ion electrolytes and facilitates their movement through one-dimensional nanochannels, potentially paving the way for safer solid-state lithium-ion batteries. The findings are detailed in a paper published in the Journal of the American Chemical Society.
The study was led by Yu Zhong, an assistant professor of materials science and engineering, whose lab focuses on synthesizing soft and nanoscale materials aimed at enhancing energy storage and sustainability technologies. Traditional lithium-ion batteries utilize liquid electrolytes, which can lead to the formation of spiky dendrites that may cause short circuits or, in extreme cases, explosions. Although solid-state batteries are safer, they present challenges, primarily due to slower ion movement through solid materials.
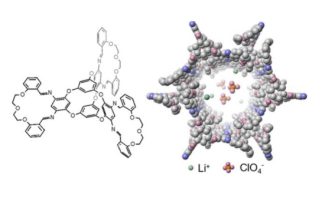
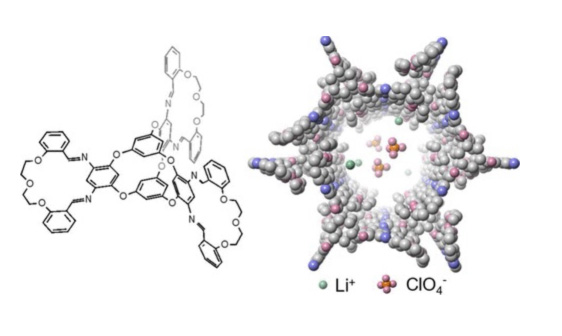
To address these issues, Zhong and his team aimed to create a new crystal that is sufficiently porous to allow ion movement through an efficient pathway while ensuring weak interactions between the lithium ions and the crystal structure to prevent sticking. The researchers fused two distinct molecular structures—macrocycles and molecular cages—into a single entity. Macrocycles consist of rings containing 12 or more atoms, while molecular cages are multi-ringed compounds.
Wang, a member of the research team, successfully combined these components, positioning a molecular cage at the center with three macrocycles extending outward like arms. The resulting macrocycle-cage molecules utilize hydrogen bonds and interlocking shapes to self-assemble into complex, three-dimensional crystals with nanopores. These crystals feature one-dimensional channels that provide an optimal pathway for ion transport, achieving a record ionic conductivity of 8.3 × 10^-4 siemens per centimeter.
In addition to their application in lithium-ion batteries, this innovative material may also be useful for separating ions and molecules in water purification processes and for creating mixed ion-electron conducting structures for bioelectronic circuits and sensors. Zhong noted the novelty of leveraging the unique geometries of these two molecular types to guide the self-assembly of more intricate structures, indicating potential for future research in developing various nanoporous materials.
The study was supported by Cornell Engineering’s Engineering Learning Initiatives and utilized resources from the Cornell Center for Materials Research and the Columbia University Materials Research Science and Engineering Center, both backed by the National Science Foundation’s Materials Research Science and Engineering Center program.